In the process of using carbon-based adsorbent to treat VOCs, whether using low pressure water vapor desorption or nitrogen desorption, the desorption medium is heated to a certain temperature, and the adsorbent is desorbed. When water steam desorption is used, water steam is generally heated to 100 ° C, mainly in order to use the latent heat of water, and there is no need to consider the pressure problem of equipment; When nitrogen desorption is used, the heating temperature can be selected, and when the temperature exceeds 100 ° C, the pressure problem of the equipment does not need to be considered. At present, the choice of desorption temperature is generally a rough method: no matter what substance is desorbed, the temperature of water vapor is generally set at 100℃ or slightly higher; Nitrogen is determined by the nature of the desorption material. Therefore, there are often misunderstandings in the choice of desorption temperature: 1) for the desorption temperature of a volatile organic matter, it is generally believed that in order to desorption these substances from the adsorbent, the desorption temperature must be higher than the boiling point of the substance; 2) Due to the misunderstanding of understanding, it is wrong to use high temperature desorption when it should not be used, which not only fails to achieve the ideal effect, but also causes energy waste.
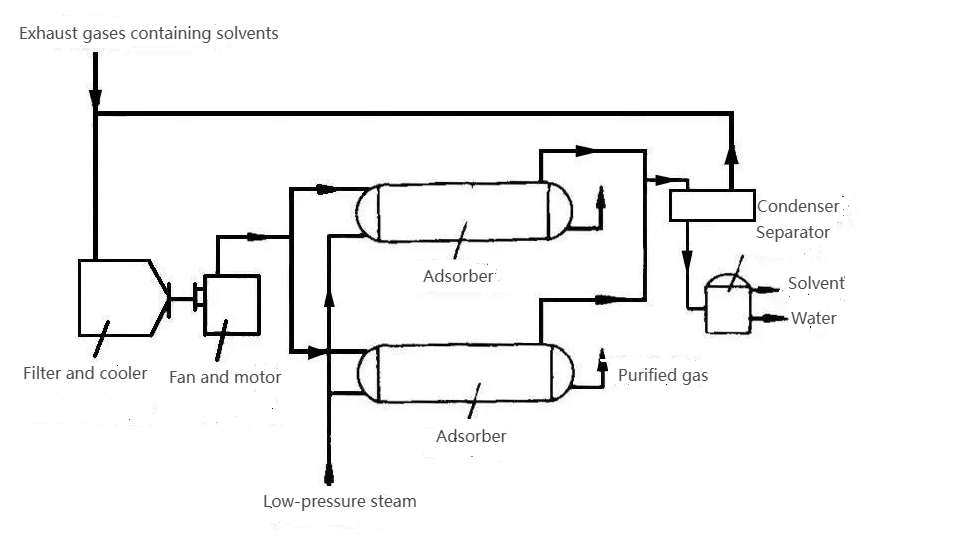
The process flow of VOCs treated by adsorption method is shown in the following figure.
General desorption methods used to govern VOCs
1. Temperature desorption
The method of desorption in which the adsorbent molecules escape from the solid adsorbent by increasing the temperature is called temperature desorption. Temperature desorption uses water vapor, hot inert gas (such as nitrogen), hot flue gas or inductive heating.
2. Decompression desorption
Decompression desorption, also known as evacuation desorption, is a desorption method to reduce the pressure around the saturated adsorbent and make the adsorbent on it escape. After pressure reduction, the partial pressure of adsorbed substance in the gas phase decreases, and the adsorption amount in equilibrium with it also decreases, and the adsorbed substance is desorbed.
3. Displacement desorption
The method of replacing the original adsorbent with a substance that has stronger affinity with the adsorbent than the original adsorbent under desorption conditions is called displacement desorption.
4. Purge and desorption
The bed is purged by a gas (such as an inert gas) that is not adsorbed by the adsorbent and the adsorbent is desorbed, which is called purging desorption.
In practical applications, several desorption methods are often combined, such as the use of water steam desorption, which has the effect of heating and purging at the same time.
VOCs Desorption condition
The desorption temperature and efficiency of some volatile organic compounds that can be observed in engineering practice are shown in the following table.
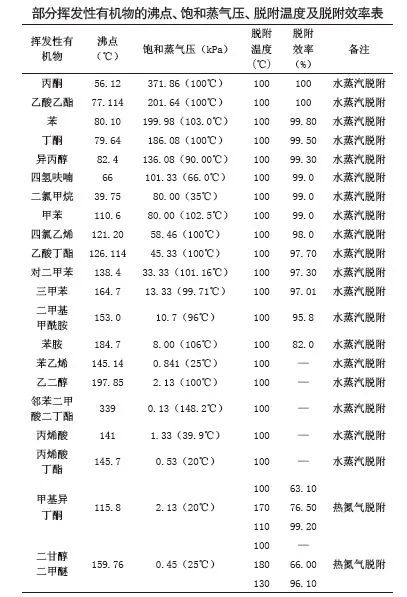
As can be seen from the above table:
(1) The desorption temperature is basically unrelated to the boiling point of the substance. Taking tritoluene as an example, its boiling point is 164.7℃, but it can be well desorbed by using water vapor at 100℃ (the desorption rate is 97.01%). For acrylic acid, which has a much lower boiling point than it (boiling point 141 ° C), the use of water vapor at 100 ° C for desorption has no effect.
(2) Looking at the various substances in the above table, all substances with saturated vapor pressure above 10.0kPa can be well desadsorbed by water vapor at 100 ° C. Substances with low saturated vapor pressure, such as styrene (0.841 at 25 ° C), dibutyl phthalate (0.13 at 148.2 ° C), butyl acrylate (0.53 at 20 ° C), etc., although the boiling point is much lower than tritylene, but because of their low saturated vapor pressure, the use of 100 ° C water vapor still cannot desorbed them.
It can be concluded that the desorption temperature of a substance has nothing to do with the boiling point, but is closely related to its saturated vapor pressure.
(3) Some substances are difficult to desorption because their saturated vapor pressure is very low. Thus, it can also correct the wrong understanding that the reason why styrene is difficult to desorption is attributed to the polymerization reaction of styrene on the surface of the adsorbent.
(4) For substances that are difficult to desorption, when hot nitrogen desorption is used, the higher the temperature, the more thorough the desorption, and the higher the desorption temperature, the lower the desorption efficiency. As shown in the table, when hot nitrogen was used to desorption methyl isobutanone (boiling point 115.8℃, saturated vapor pressure at 20℃ is 2.13kPa), it was found that when the temperature rose to 100℃, the desorption rate was only 63.10%. In order to improve the desorption rate, the nitrogen temperature was increased to 170℃, and the desorption rate reached 76.50%. At this time, it is meaningless to consider further warming, and the temperature is tried to decrease, and it is found that the desorption rate gradually increases. When the temperature dropped to 110℃, the desorption rate reached a peak of 99.20%.
Therefore, it is concluded that the higher the temperature is, the more complete the desorption is when it is desorption difficult to desorption, but the higher the desorption temperature will decrease the desorption efficiency. In case of such problems, the appropriate desorption temperature should be carefully selected through experiments to obtain the best desorption efficiency.
Analysis of VOCs desorption effect
(1) The relationship between desorption temperature and saturated vapor pressure. From the principle of desorption, the fundamental reason for the desorption of adsorbent from the surface of the adsorbent is that the adsorbent molecule must overcome the attraction of the adsorbent surface to it and increase its driving force from the surface.
That is to say, in order to desorption the adsorbent molecule from the adsorbent surface, it must be given energy or impetus so that it can “evaporate” from the adsorbent surface into the adsorbent pore, thus entering the gas phase body.
In the usual desorption method, heat desorption is to provide energy to increase the kinetic energy of molecules. Scavenging and step-down (vacuum) desorption are all in order to reduce the partial pressure of the exhaust gas molecules in the adsorbent pore, that is, the vapor pressure, causing a concentration difference to the exhaust gas, thus providing a driving force for the transfer of exhaust gas molecules from the adsorbent surface to the gas phase, the greater the driving force, the faster the desorption rate of exhaust gas molecules. Therefore, it is not difficult to understand from this theory that the desorption temperature of the adsorbed material is directly related to its saturated vapor pressure, and has nothing to do with its boiling point.
(2) When some substances with low saturated vapor pressure are desorbed, the temperature is too high, but the desorption rate will decrease. According to the classification of adsorption, it can be divided into physical adsorption and chemical adsorption. The bond energy formed by physical adsorption is only in the range of van der Waals force, that is, the maximum is only about 80kJ/kmol, while the adsorption bond force of chemical adsorption can reach more than 400kJ/kmol.
In the adsorption of substances, there is often a phenomenon: when the temperature is low, it is physical adsorption, and if the temperature is increased, it may be transformed into chemical adsorption. That is to say, when the desorption temperature is too high, the existing physical adsorption state may be transformed into a chemisorption state, making the bond energy of the adsorption bond greatly increase, so it is not easy to desorption down. This is why the temperature is too high, but the material desorption rate decreases.
Of course, in order to fully understand this problem, only the bond energy of the adsorption bond in two states can be determined. However, it is still difficult to determine the bond energy of adsorption bonds at present. Although some people can determine the bond energy of chemical bonds of some substances by using synchrotron radiation photoionization method, it has not been reported whether this method can determine the bond energy of adsorption bonds well.
Suggestions for determination of desorption temperature
(1) For substances with saturated vapor pressure > 10kPa, in principle, water vapor at 100 ° C can be used for desorption; However, from the perspective of energy conservation, it is recommended to saturated vapor pressure and low boiling point (such as < 70℃) of substances, such as: acetone: boiling point 56.1℃, saturated vapor pressure 2371.86kPa (100℃); Tetrahydrofuran: boiling point 66℃, saturated vapor pressure 101.33kPa (66.0℃); Methylene chloride: Boiling point 39.75℃, saturated vapor pressure 80.00kPa (35℃), etc., it is recommended to use lower temperature nitrogen for desorption, so that not only can reduce the temperature of desorption agent, but also need not use very low temperature condensate for condensation separation when condensing the mixed gas after desorption (such as dichloromethyl methane needs to use 7℃ cold water for condensation separation). You can save energy. Due to the use of nitrogen desorption, the problem of condensate treatment is eliminated.
(2) When using high temperature desorption for substances with low saturated vapor pressure, it is also necessary to use appropriate temperature for desorption, so that both high desorption efficiency can be received and energy saving can be achieved.
Of course, for the choice of desorption temperature of various substances, there is no readily available data to query, but also need to conduct repeated experiments to determine the initial, and then conduct economic feasibility analysis in order to finally determine whether the selected desorption temperature is appropriate.